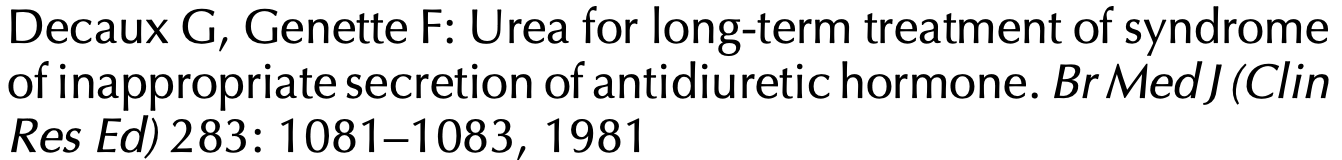Information for Pharmacists

Click below to view two clinical update videos presented by: Harold Szerlip MD, MS (ED) FACP, FASN FNKF, CCRP. Director, Division of Nephrology, Baylor University Medical Center, Dallas TX.
Hyponatremia: Clinical update on inpatient treatment.
Hyponatremia: Clinical update on outpatient treatment.
November 2018 CJASN published clinical study on the use of ure-Na to treat hyponatremia in the inpatient setting.

- 15g USP Urea per pouch
- Does not contain any NaCl
- Easily mixes with water or juice
- Clinically Studied & Guideline supported (See 'Clinical References' section for more information)
- Safe, effective and cost sensitive
- ure-Na is pronounced: you-ree-nah
Distribution Partners

ure-Na code used in place of an NDC
62530-0000-11
ure-Na wholesale availability
ure-Na is available for wholesale purchase from McKesson, Cardinal, AmerisourceBergen, Morris and Dickson and Value Drug. See FAQ section below for item numbers.
Ure-Na
Ure-Na is available in a box with 8 sachets containing 15g USP urea per sachet.


Mechanism of Action of urea for the treatment of hyponatremia
Urea normalizes serum sodium by inducing osmotic excretion of free water without associated electrolyte depletion. Urea also ameliorates hyponatremia in SIADH by a more specific effect, diminishing the natriuresis in association with increased medullary urea content.
European Clinical Practice Guideline on the Diagnosis & Treatment of Hyponatremia
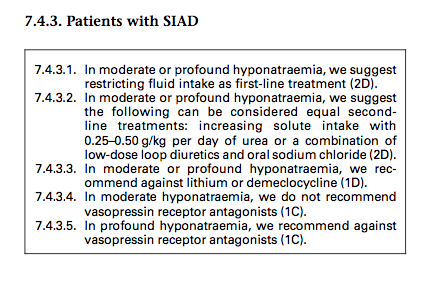
Dosing chart based off of the above European Guideline dose range

mOsm comparison of urea vs. NaCl
15g of urea found in 1 sachet of ure-Na equals 250 mOsm, equal to 14.7 500mg NaCl tablets or 7.35 1g NaCl tablets.30g of urea found in 2 sachets of ure-Na equals 500 mOsm, equal to 29.4 500mg NaCl tablets or 14.7 1g NaCl tablets.
Discharging or initiating a patient on ure-Na

Ure-Na can be ordered from most retail pharmacies for next day arrival. Most retail pharmacies receive a daily shipment Monday - Friday from their pharmaceutical wholesalers where ure-Na is stocked.
We recommend the prescription or order for ure-Na includes the NDC # or UPC # so it can be found in the pharmacy ordering system.
NDC# 62530-0000-11
UPC# 862530000116
We recommend that a patient be discharged on two or three days worth of ure-Na. If a patient is discharged on a Friday, the next time most retail pharmacies will be able to get ure-Na will be Monday with their next wholesale delivery.
Ure-Na can also be ordered on this website or by calling 1-844-980-9933.
More information can be found in the Buy ure-Na section of ure-na.com
Published data on the use of ure-Na in the inpatient setting
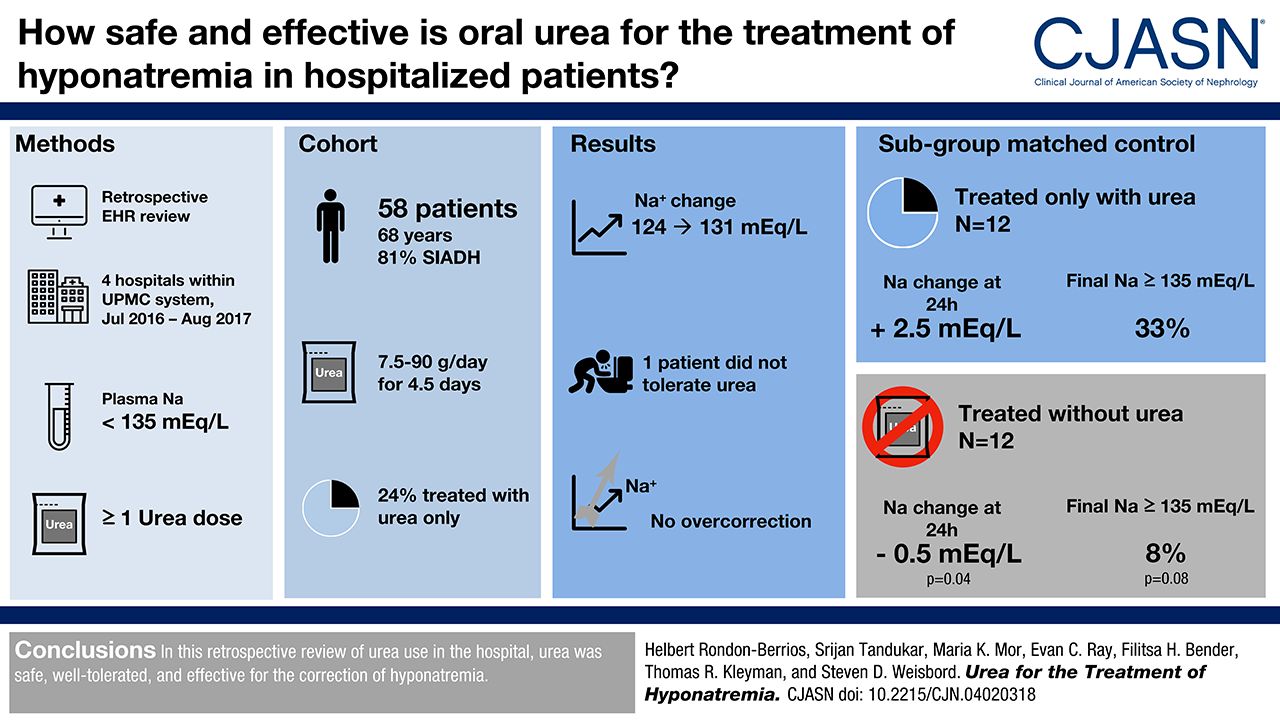
Long term durability of urea treatment response
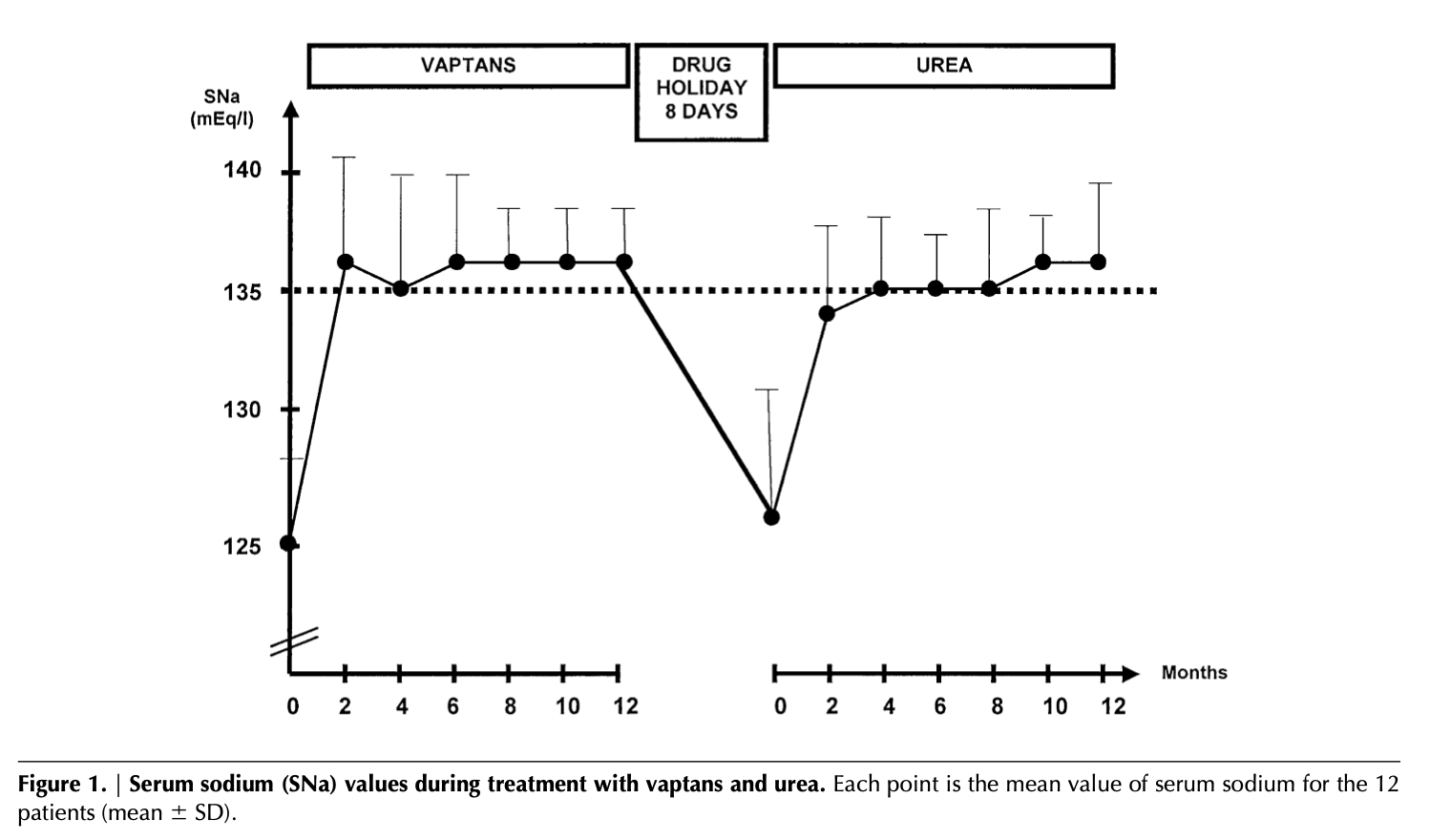

Patients not appropriate for ure-Na
Patients with hypovolemic hyponatremia
Patients with hyponatremia associated with adrenal insufficiency
Patients with hyperammonemia
ure-Na: BUN and Serum Creatinine
Urea is produced normally in the body by the liver. As such, it is considered safe (GRAS designation by the FDA), and does not carry nephrotoxic or hepatotoxic potential. Since the blood test for BUN measures Blood Urea Nitrogen levels, it is expected that when ure-Na is administered, an increase in BUN levels may be seen. However, this isolated increase in not indicative of kidney function, and is similar to what may be seen when individuals consume a high protein diet with production of additional amounts of urea by the liver.
Below are three data sets that report pre and post treatment serum urea or BUN as well as serum or blood creatinine.
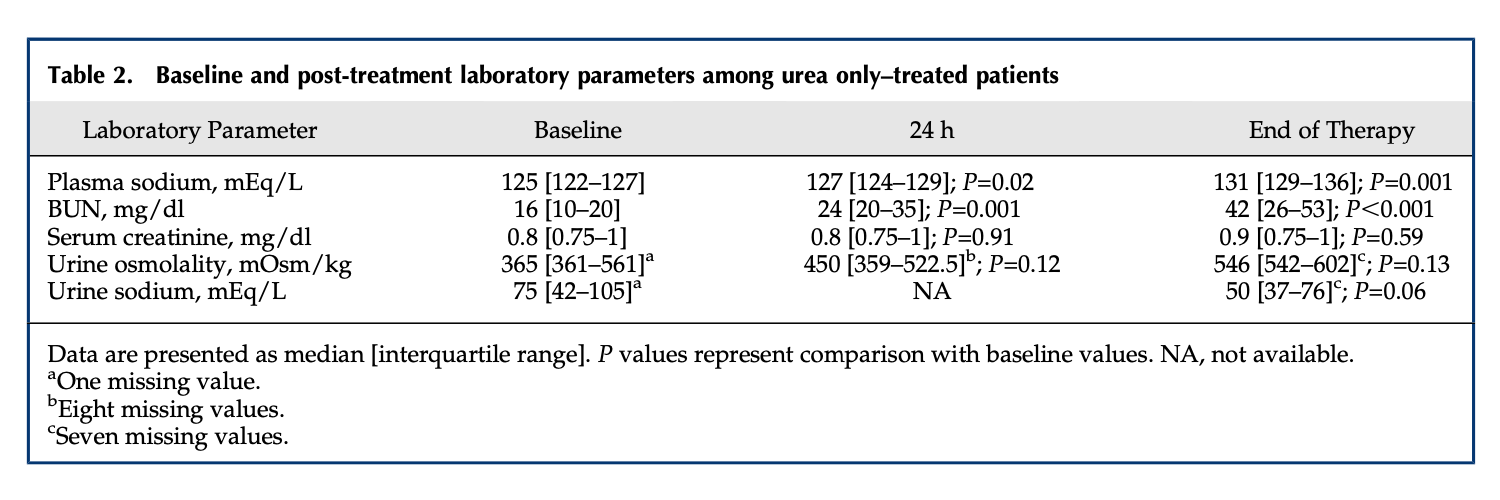

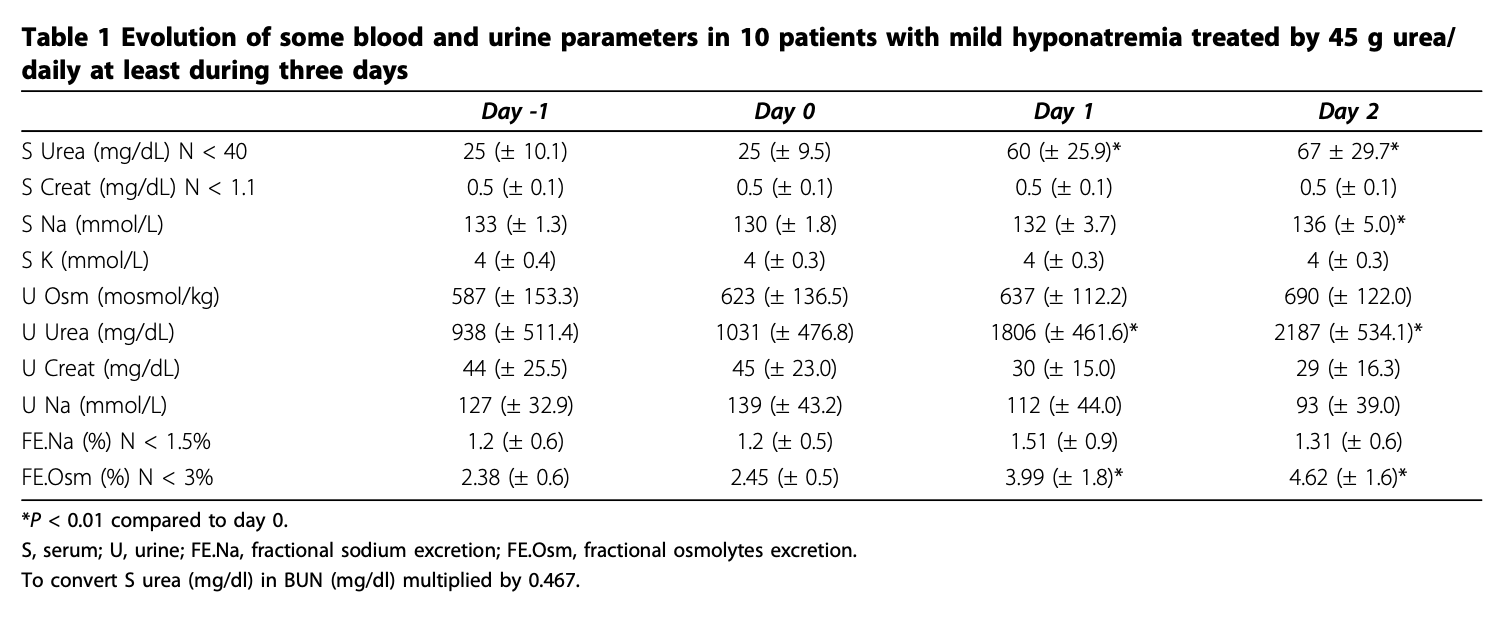

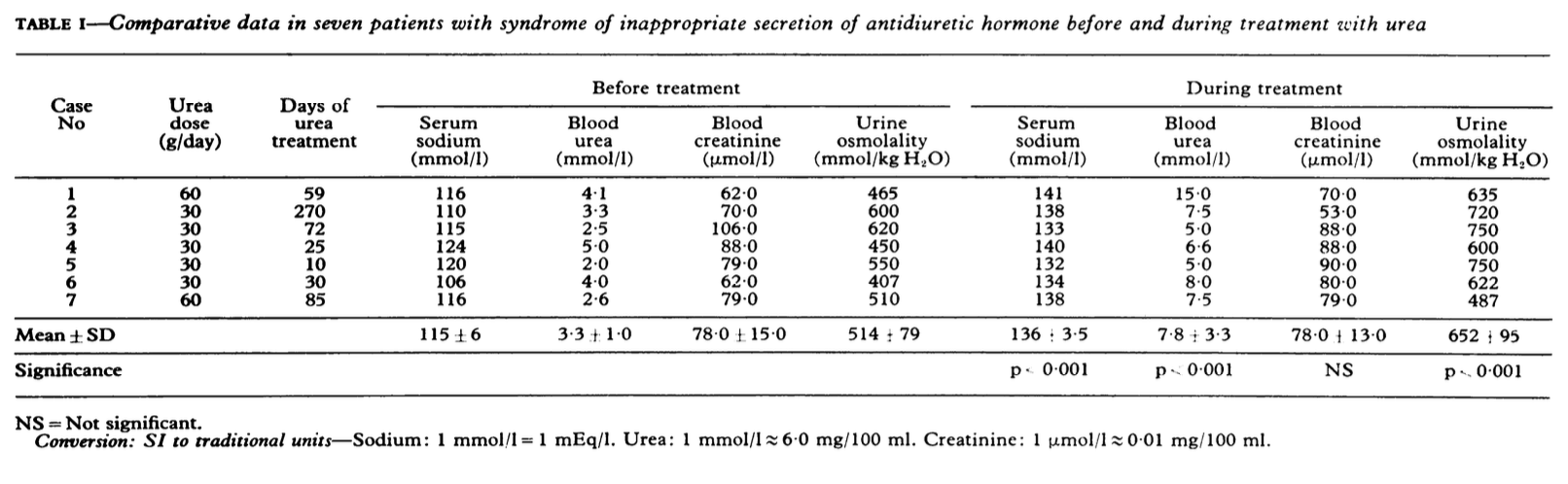
This data has treatment lengths up to 270 days
Formulary Review of ure-Na
If you are conducting a formulary review of ure-Na and would like a copy of the AMCP Dossier, please email us at [email protected] or scroll down and click on the Contact Us button at the bottom of the page.
ure-Na ingredients
Ingredients in descending order: synthetically derived urea powder, natural flavors, maltodextrin, citric acid, calcium silicate, sucralose.
Frequently Asked Questions
A: ure-Na is currently available through McKesson, Cardinal, AmerisourceBergen and Morris & Dickson. McKesson item#: 3572344. Cardinal item# 5286018. AmerisourceBergen item# 10173267 or 619114. Morris & Dickson item# 942680. If ure-Na isn't available in the DC that you pull from, please contact your Wholesale Rep and ask them to bring ure-Na into your DC. They may ask you for an estimated use so they can stock the DC appropriately. If you would like us to reach out to the DC you pull from please call us at 1-844-980-9922, option #4.
A: For coverage under Medical Benefit the HCPCS code typically used is: A9999
A: ure-Na is USP urea with a proprietary flavor and taste masking formula, which makes it palatable. It is used for the management of hyponatremia. It is known that hyponatremia is rarely a sodium deficiency, but rather an excess of water in the blood relative to sodium. Urea normalizes blood sodium levels by inducing water loss in the urine. This is accomplished without a risk of loss of important electrolytes like potassium, calcium and magnesium. Treatment with urea is particularly helpful in cases of SIADH (Syndrome of inappropriate ADH Secretion).
A: ure-Na is a medical food used to treat euvolemic and hypervolemic hyponatremia, including SIADH.
A: Guidelines from the European Society of Endocrinology, The European Society of Intensive Care Medicine, and The European Renal Association- EDTA support the use of urea for the management of hyponatremia:(click for link)
http://www.eje-online.org/content/170/3/G1.full.pd... UpToDate has also reviewed the use of urea in hyponatremia and has recommendations on dosing.(click for link) http://www.uptodate.com/contents/overview-of-the-treatment-of-hyponatremia-in-adults
A: In a comparison study of urea versus vaptans , urea had similar efficacy raising serum sodium to 135 mEq/L. (Soupart A: Efficacy and Tolerance of Urea Compared with Vaptans for Long-Term Treatment of Patients with SIADH. CJASN Vol 7 May, 2012.) In another study of the use of urea in an ICU setting, urea raised serum sodium 4 mmol/L the first day of use, and 7 mmol/L in 48 hours. (Decaux et al: Treatment of euvolemic hyponatremia in the intensive care unit by urea., Critical Care 2010 14:R184.) This rate of rise is very important from a safety standpoint, because too rapid a rise of serum sodium levels can be dangerous, and even life threatening. The above study showed that the rise was within the recommended levels of raising serum sodium levels less than 9mmol/L in the first 24 hours.
A: Yes, please email us at [email protected] or fill out the contact form in the Contact Us section and we'll send you the dossier.
A: The risks of taking urea are very low. No long term toxicity has been seen. There is a very low risk of hypernatremic dehydration if your patients thirst center is intact. (Decaux G: Urea for long term treatment of syndrome of inappropriate secretion of antidiuretic hormone. BMJ Vol 283 24 Oct 1981.)
A: A 15g dose of ure-Na easily mixes into solution in 3-4 ounces of water or juice. As you know, hyponatremia can be a difficult condition to manage. We recommend that ure-Na be taken under medical supervision and that the amount of water or juice that ure-Na is mixed with is determined by the health care provider.
A: Each 15g dose of urea in a serving of ure-Na is individually packaged in easy to open pouches. There are 8 pouches or doses per box.
A: As each patients therapeutic needs are different, this determination will need to be made by the health care provider.
A: The European guidelines* on the use if urea recommend a dose of 0.25-0.50g/Kg (a 140lb person then would take 15.75 - 31.5g) UpToDate discusses a daily dose of 30g.
A: Please see the Buy Now section found on the home page for purchasing options.
Please see the Insurance section found on the home page for more in-depth information on insurance coverage.
ure-Na may be covered by health insurance under the medical benefit through prior authorization. If ure-Na is not covered by insurance, it may be a tax deductible qualified medical expense.
Dosing for a Prior Auth: ure-Na is packaged in 8 count boxes. If a patient is on 15g/day the dispensing request on the PA will be ure-Na 8 count x 4. If the patient is on 15g/BID (30g/day) the dispensing request will be ure-Na 8 count x 8. The billing code (HCPCS) for coverage under medical benefit is A9999.
If ure-Na is not covered by health insurance, it can be ordered at most retail pharmacies, or at
www.ure-na.com.
A: The European Guidelines* recommend the use of urea in moderate to profound hyponatremia.
A: Mild GI upset has been seen in a minority of patients taking ure-Na.
A: Urea is not nephrotoxic or hepatotoxic like other therapeutic options for the management of hyponatremia.
A: There is data on the use of urea in doses of 15g and 30g per day for 1 year that show no toxicity. (Soupart A:Efficacy and Tolerance of Urea Compared with Vaptans for Long Term Treatment of Patients with SIADH. CJASN Vol 7 May, 2012)
A: Unlike supplements a medical food, as defined in section 5(b)(3) of the Orphan Drug Act (21 U.S.C. 360ee(b)(3)), is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” Medical Foods, including ure-Na can be purchased OTC without a prescription. Ure-Na is made in the USA in an FDA inspected facility under cGMP guidelines.
A: The FDA classifies urea as GRAS or Generally Recognized As Safe. This is a designation that a chemical or substance added to food is considered safe by experts, and so is exempted from the usual Federal Food, Drug and Cosmetic Act (FFDCA) food additive tolerance requirements.